Arrange the Following Bonds in Order of Increasing Polarity
Arrange the following bonds in order of increasing bond polarity. Cl-S Cl-P Cl-Si Cl-Cl A Cl-S.
Solved Arrange The Following Bonds In Order Of Increasing Chegg Com
Arrange the bonds in each of the following sets in order.

. CeC - H N - H O - H F - H Concept. Arrange the following bonds in order of increasing polarity using the periodic trends in electronegativity. O-cl c-cl h-cl and f-cl.
Arrange the following in order of increasing ionization energy. Arrange the following bonds in order of increasing polarity. First calculate the electronegativity difference E between atoms in each bond.
Cl-Cl E Cl-Cl. Using the table of electronegativities provided arrange the following bonds in order of increasing ionic character. F-F F-C F-O F-N lower first higher last F-F F-O F-N F-C Given that 4NH₃g 5O₂g 4NOg 6H₂Og if 63 moles of NH₃ react with sufficient oxygen how many moles of NO will be formed.
Arrange the following bonds in order of increasing polarity. Therefore the bond between H and Cl is polar covalent. Cl-Si B Cl-S.
Arrange the following compounds in order of increasing dipole moment. Bond Parameters - Polarity of Bonds. I Toluene II m dichlorobenzene III o dichlorobenzene IV p dichlorobenzene.
Arrange the following bonds in order of increasing polarity. Arrange the following molecules in order of increasing bond polarity highest bond polarity at the bottom. Cl-S C Cl-Si.
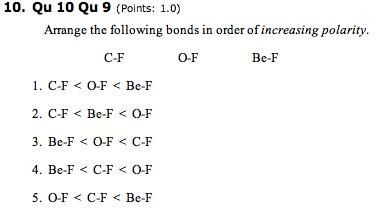
Then arrange the bonds from smallest E value to greatest _E value. C-C C-F C-Cl. A CF OF BeF.
P - H H - O N - H H - F. C- CC-I C-CI C-F B. SOLVEDArrange the following molecules in order of the increasing polarity of their bonds.
Polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end. Video Player is loading. 1C-0 2P-S 3C-S 4Cl-Br 5Br-F 1 See answer Advertisement Advertisement rexsagorio rexsagorio Answer.
The shape of a BF3 molecule is. Arrange the following bonds in order of increasing polarity. A OH CH HH FH.
Si-P 21 - 18 03. Ozone forms when the bond in O2 breaks and each O atom reacts with another O2 molecule. Which of the following would be a polar molecule.
When electronegativity difference is greater than 04 and less than 17 then bond between the two atoms is a polar covalent bond. Arrange the following bonds in order of increasing polarity. Determine whether polar or non-polar the following paired atomsThenarrange the following bonds in order of increasing polarity.
When electronegativity difference is 17 or greater than the bond formed is ionic in nature. Explain the trend that bond formation does to atomic order by selecting all those that apply. 7 connect hw Without stratospheric ozone O3 harmful solar radiation would cause gene alterations.
Place the least polar bond at the top of the list Pd-H. Arrange the given bonds in increasing order of polarity. C-F C-C C-CI C-I 1 н 1008 3 4 Li Ве 69419012 11 12 A.
The anion in an ionic compound whose name ends in ide is. To the above given difference in the electronegativities the order of increasing ionic character is. P - H H - O N - H H - F.
Both polar bonds and an unsymmetrical arrangement. 1C-0 25-35 1 POLAR. Cl-Cl D Cl-Cl.
B OCl SBr CP. So when the bond is between the same element the polarity is 0 such as H-H. C-F C-CI C-I C-C Polarity is based on the DIFFERENCE in EN C-C is the least polar C-F is the most polar since.
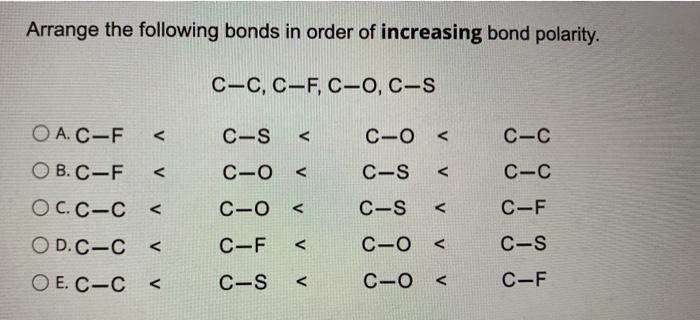
Arrange the given bonds in increasing order of polarity. Arrange the following bonds in order of increasing polarity least to most polar C-O C-C C-N C-F. A mathrm HCl b mathrm PH_ 3 c mathrm H_ 2 mathrm O d mathrm CF_ 4 Problem.
A H Cl b C-O c N-F d Si- I and e O O. Ammonia N H 3 and phosphorus trihyd. Arrange the following bonds in order of increasing polarity.
C- FC-C C-Cl. Therefore electronegativity difference of the given species is as follows. The lewis symbol for Mg atom is.
H has a low electronegativity then it comes C O and F so the order of increasing of polarity is. It is destroyed by reaction with Cl atoms formed when the CCl. C CS BF NO.
H-Cl H-I H-Br H-F.

Solved Arrange The Following Bonds In Order Of Increasing Polarity 1 Point H F N F F F H Fi F F N F N F F F H F F F N F H F
Solved Arrange The Following Bonds In Order Of Increasing Chegg Com

Solved Question 13 Arrange The Following Bonds In Order Of Chegg Com
Comments
Post a Comment